Writing the perfect resume for a Regulatory Affairs Specialist job can be a daunting and difficult task. With the ever-changing landscape of regulations and laws, it is important to have a resume that clearly outlines your experience and qualifications within the field of regulatory affairs. This guide will provide you with tips on how to write a strong resume, including what to include, what to leave out, and some resume examples that can help you stand out as a Regulatory Affairs Specialist. With this guide, you will be able to craft a resume that will help you land the job of your dreams.
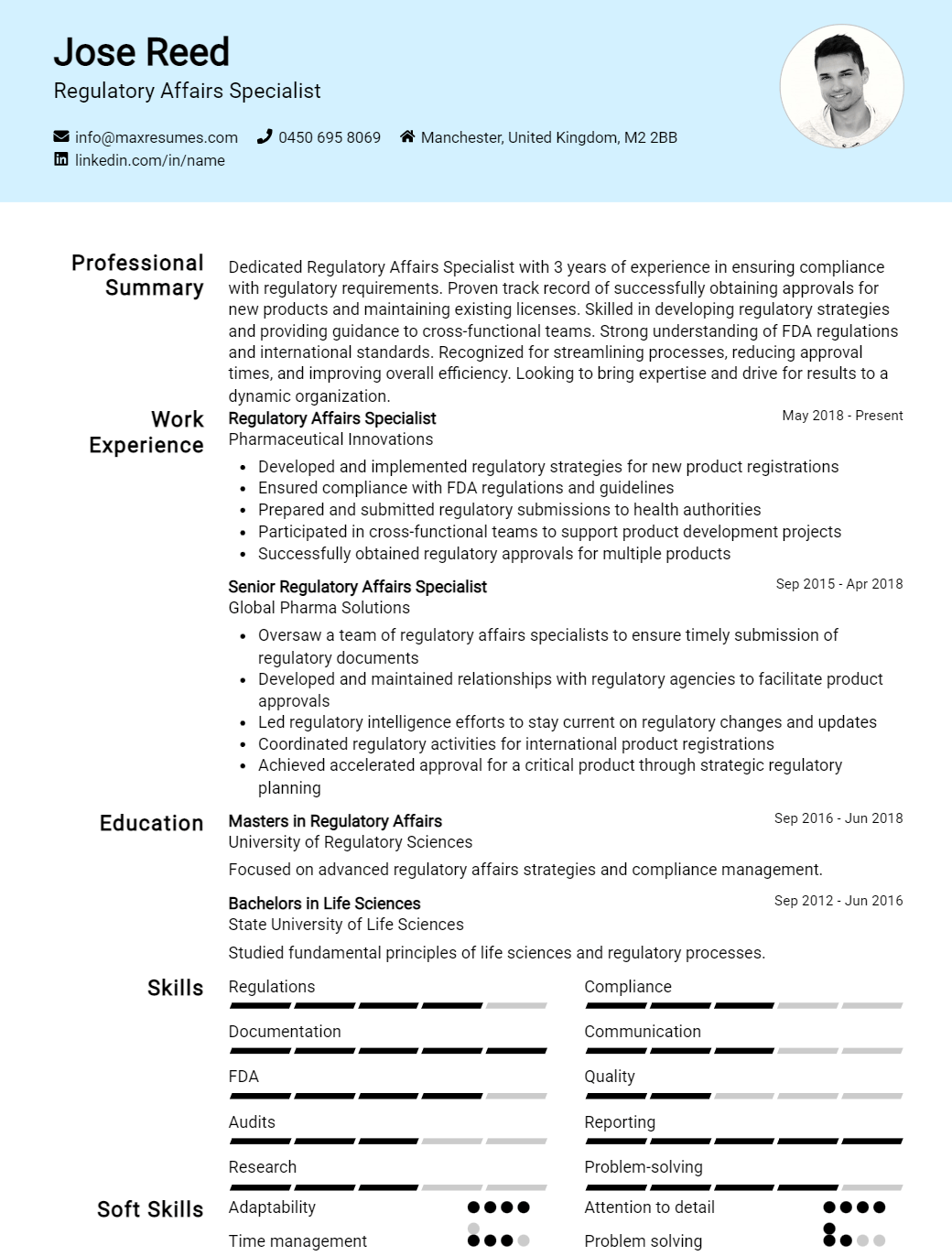
Regulatory Affairs Specialist Resume Sample
If you didn’t find what you were looking for, be sure to check out our complete library of resume examples.

Start building your dream career today!
Create your professional resume in just 5 minutes with our easy-to-use resume builder!
Regulatory Affairs Specialist Resume Examples
John Doe
Regulatory Affairs Specialist
123 Main Street | Anytown, USA 99999 | Phone: (123) 456-7890 | Email: john.doe@email.com
Highly organized and detail- oriented Regulatory Affairs Specialist with 5+ years of experience in developing, implementing and managing regulatory strategies for medical and pharmaceutical products. Proven track record of leading successful regulatory submissions and ensuring compliance with applicable regulations in order to obtain regulatory approval. Possess effective communication and interpersonal skills and experience in working closely with internal and external stakeholders.
Core Skills:
- Regulatory strategy development
- Regulatory submission & compliance
- Regulatory risk management
- Project management
- Interpersonal & communication skills
- Attention to detail
Professional Experience:
Regulatory Affairs Specialist, ABC Pharmaceuticals, Inc.
October 2018 – Present
- Develop, implement, and manage regulatory strategies for medical and pharmaceutical products.
- Prepare and submit regulatory dossiers and documents for regulatory approval in order to obtain market entry of products.
- Manage and coordinate regulatory activities with internal and external stakeholders and ensure compliance with applicable regulations.
- Review advertising and promotional materials and support product labeling activities.
- Update internal stakeholders on regulatory changes and provide project management support.
Regulatory Affairs Associate, XYZ Technologies, Inc.
March 2017 – October 2018
- Prepared and submitted applications for regulatory approval to various international regulatory bodies.
- Monitored and maintained regulatory standards to comply with federal and international regulations.
- Assisted in the development of regulatory strategies for clinical development, marketing and labeling activities.
- Maintained regulatory databases and collected information for regulatory filings.
- Conducted research to identify and develop strategies for regulatory processes and procedures.
Education:
Master of Science in Regulatory Affairs, University of Miami
Bachelor of Science in Pharmaceutical Sciences, University of Miami
Regulatory Affairs Specialist Resume with No Experience
Regulatory Affairs Specialist with no experience looking to help pharmaceutical companies meet the legal and regulatory standards of the industry. Possess a keen understanding of regulations and strive to keep the company compliant at all times. Experienced in data analysis, researching, and report writing.
Skills
- Strong research and analysis skills
- Excellent communication, interpersonal and organizational skills
- Strong problem- solving and decision- making abilities
- Familiarity with relevant laws and regulations
- Ability to work collaboratively and independently
- Proficient in Microsoft Office Suite, Adobe Acrobat, and database management programs
Responsibilities
- Analyze and interpret regulations and guidelines from federal and state agencies
- Research, develop and implement regulatory strategies
- Guide staff on regulatory compliance matters
- Maintain current knowledge of regulations and industry best practices
- Investigate and resolve regulatory inquiries
- Create and submit regulatory documents to the necessary agencies
- Ensure compliance with local, state, and federal regulations
Experience
0 Years
Level
Junior
Education
Bachelor’s
Regulatory Affairs Specialist Resume with 2 Years of Experience
I am an experienced Regulatory Affairs Specialist with over two years of experience in the highly regulated fields of medical device and pharmaceutical products. I am highly competent in preparing regulatory documentation, ensuring compliance with both national and international regulations, and managing the entire product life- cycle. My diverse skill- set has enabled me to partake in a number of successful product development projects for top- tier companies. I am a diligent worker and strive to provide exceptional results in all tasks and projects assigned.
Core Skills:
- Regulatory Submission Preparation
- Product Life- Cycle Management
- Regulatory Compliance
- Technical Writing
- Project Management
- Knowledge of National and International Regulations
- Time Management
- Problem Solving
- Attention to Detail
Responsibilities:
- Preparing regulatory documentation and submissions based on national and international regulations
- Maintaining and updating regulatory dossiers to ensure compliance with current regulations
- Coordinating with internal and external stakeholders to ensure project completion
- Fostering and managing relationships with regulatory authorities
- Developing and implementing regulatory strategies to ensure regulatory compliance
- Investigating and responding to regulatory inquiries
- Analyzing and interpreting regulatory data and trends
- Assisting with product labeling, packaging, and advertising reviews
Experience
2+ Years
Level
Junior
Education
Bachelor’s
Regulatory Affairs Specialist Resume with 5 Years of Experience
Dynamic Regulatory Affairs Specialist with 5+ years of experience in the medical device and pharmaceutical industries. Skilled in obtaining and renewing product registrations and licenses, as well as writing and submitting device and drug registrations and applications. Strong communicator capable of handling complex regulatory requirements, interactions with stakeholders, and working in highly regulated industries. Dedicated to ensuring compliance of products and services with local, national, and international regulations.
Core Skills
- Regulatory compliance
- Documentation
- Regulatory strategy
- Risk assessment
- Regulatory submissions
- License renewals
- Change control management
- Scientific writing
Responsibilities
- Managed the regulatory requirements of FDA, ISO, MDD, and 510(k) submissions.
- Developed regulatory strategies and tracked compliance documents for medical device products.
- Wrote, submitted, and obtained product registrations and licenses in several countries.
- Maintained change control management program and monitored product compliance.
- Assessed product risks, investigated non- conformances, and developed corrective action plans.
- Ensured the maintenance of product registrations and renewals with regulatory agencies.
- Prepared and reviewed scientific documents for regulatory submissions.
- Liaised with internal and external stakeholders for regulatory affairs.
Experience
5+ Years
Level
Senior
Education
Bachelor’s
Regulatory Affairs Specialist Resume with 7 Years of Experience
I am a highly organized and detail- oriented Regulatory Affairs Specialist with 7 years of experience in the regulated healthcare industry. I have extensive experience in medical device and pharmaceuticals, as well as biologics, including obtaining regulatory approvals, developing and maintaining regulatory strategies, and ensuring compliance with applicable regulations. I possess a strong ability to work collaboratively with colleagues and other stakeholders to ensure successful product launches, as well as excellent communication, analytical, and problem- solving skills.
Core Skills:
- Strong knowledge of regulations and experience in medical device and pharmaceuticals, biologics, and biologics
- Excellent communication, analytical, and problem- solving abilities
- Ability to work collaboratively with colleagues and other stakeholders
- Ability to lead product launches and ensure successful outcomes
- Knowledge of global regulatory requirements and procedures
Responsibilities:
- Developed and maintained regulatory strategies for product launches
- Obtained approvals from international and local regulatory bodies for medical device and pharmaceuticals, biologics, and biologics
- Ensured compliance with applicable regulations and global regulatory requirements
- Provided regulatory advice to internal and external stakeholders on product development and launch initiatives
- Collaborated with cross- functional teams such as clinical research, marketing, legal, and quality assurance
- Conducted gap analyses to identify areas of non- compliance
- Reviewed product labeling and advertising content for compliance
- Developed regulatory plans and timelines for product launches.
Experience
7+ Years
Level
Senior
Education
Bachelor’s
Regulatory Affairs Specialist Resume with 10 Years of Experience
Highly motivated, detail- oriented Regulatory Affairs Specialist with 10 years of experience in the healthcare sector. Experienced in managing, developing and overseeing all regulatory projects from initiation to completion. Skilled in navigating complex regulatory processes, analyzing and interpreting data and providing meaningful solutions for problems. Possess excellent organizational and communication skills, with a demonstrated history of working collaboratively with teams to ensure objectives are met.
Core Skills:
- Proficiency in regulatory affairs, quality assurance and healthcare systems
- Knowledge of healthcare laws and regulations
- Excellent problem solving, analytical and research skills
- Ability to interpret complex data and identify trends
- Strong written and verbal communication skills
- Proficiency in MS Office Suite, Adobe Acrobat and other relevant software
- Excellent organizational and coordination skills
- Demonstrated ability to work alone and in teams
Responsibilities:
- Develop and maintain regulatory plans for assigned projects
- Monitor and evaluate regulatory policies and procedures
- Analyze and interpret data and develop meaningful solutions
- Ensure compliance with applicable laws, regulations and standards
- Provide training and guidance to the team on regulatory matters
- Maintain accurate and complete records of regulatory activities
- Conduct audits of regulatory processes and procedures
- Assist in the preparation of various regulatory documents
- Communicate with applicable regulatory authorities and stakeholders
- Collaborate with other teams to ensure objectives are met
Experience
10+ Years
Level
Senior Manager
Education
Master’s
Regulatory Affairs Specialist Resume with 15 Years of Experience
Regulatory Affairs Specialist with 15+ years of experience in medical device, biologic and pharmaceutical regulations. Demonstrates expertise in Good Manufacturing Practices (GMP) and clinical trial regulations. Proven ability to develop and maintain successful relationships with regulatory bodies, internal and external stakeholders.
Core Skills:
- Extensive experience in developing regulatory strategies for medical device and pharmaceutical products
- Comprehensive knowledge of regulatory guidelines, policies, and procedures
- Proficient in international regulations and guidelines
- Outstanding communication, negotiation, and problem solving skills
- Highly analytical with excellent organizational and project management skills
Responsibilities:
- Develop regulatory strategies and plans for medical device and pharmaceutical products
- Prepare regulatory submissions to local and international regulatory authorities
- Monitor and ensure compliance with local and international regulations
- Analyze and interpret regulatory documents, policies, and procedures
- Liaise with internal and external stakeholders, regulatory bodies, and other relevant parties
- Research and analyze current and proposed regulations, industry standards, and market trends
- Prepare responses to regulatory inquiries and requests for additional information
- Manage the regulatory document control system
- Ensure all regulatory activities are completed in a timely and accurate manner
- Monitor the progress of product registrations and licenses
- Monitor and audit production for compliance with GMP and other regulatory standards
- Assist with clinical trial and post- marketing surveillance activities
Experience
15+ Years
Level
Director
Education
Master’s
In addition to this, be sure to check out our resume templates, resume formats, cover letter examples, job description, and career advice pages for more helpful tips and advice.
What should be included in a Regulatory Affairs Specialist resume?
When crafting a Regulatory Affairs Specialist resume, it is important to include the relevant skills and experience that are required to excel in the job. Your resume should be tailored to the specific job description and should include the following key elements:
- Summary: A brief introduction to your qualifications and experience that focuses on the most relevant skills and abilities that make you an ideal candidate for the role.
- Education: A list of your educational qualifications and any relevant certifications and licenses related to the role of a Regulatory Affairs Specialist.
- Work Experience: An overview of your professional experience, including any roles that demonstrate your ability to implement regulatory requirements in a professional environment.
- Skills and Knowledge: A list of the relevant technical and soft skills that you possess, such as knowledge of regulatory compliance and of laws and regulations relevant to the industry.
- Achievements: A list of any key accomplishments such as successful process improvements or implementations of regulatory standards.
- Professional Membership: Any memberships to professional associations or organizations related to regulatory compliance.
By following these guidelines, your resume will give employers a comprehensive overview of your professional qualifications and experience, allowing you to stand out from the competition.
What is a good summary for a Regulatory Affairs Specialist resume?
A Regulatory Affairs Specialist is responsible for developing and implementing regulatory strategies for an organization’s products and services. They must ensure that all products and services comply with applicable laws and regulations, and serve as the point of contact for regulatory authorities.
An ideal summary for a Regulatory Affairs Specialist resume should highlight the candidate’s experience and knowledge in the industry, as well as their ability to effectively manage regulatory functions. The summary should also demonstrate the candidate’s ability to develop and implement regulatory strategies, and their ability to work effectively with regulatory authorities. Furthermore, the summary should highlight their strong organizational and communication skills, as well as their proficiency in researching, interpreting, and implementing relevant regulations. Finally, the summary should include any other relevant experience and skills, such as FDA experience, GxP compliance experience, or experience working with medical devices.
What is a good objective for a Regulatory Affairs Specialist resume?
A Regulatory Affairs Specialist is responsible for ensuring a company’s products comply with applicable regulations. A well-written objective statement in a resume for this position should reflect the applicant’s knowledge and understanding of the industry. It should also demonstrate the applicant’s ability to work with teams and management on all regulatory matters. Here is an example of a good objective for a Regulatory Affairs Specialist resume:
- To obtain a position as a Regulatory Affairs Specialist and leverage my extensive experience in regulatory compliance and process optimization.
- To utilize my knowledge of FDA and global regulations to ensure compliance and facilitate product approvals.
- To collaborate with teams to develop and implement compliant product processes.
- To stay current with all relevant regulations and industry changes in order to ensure compliance.
- To provide strategic guidance to management on regulatory processes and drive continuous improvement initiatives.
How do you list Regulatory Affairs Specialist skills on a resume?
When writing a resume for a Regulatory Affairs Specialist, it is important to include a list of skills in order to demonstrate your qualifications for the position. These skills may include technical, regulatory and problem-solving proficiency. Make sure to include any certifications or education related to regulatory affairs that make you a more attractive candidate.
Below are some skills you should consider including when listing your qualifications on your resume:
- Knowledge of Federal and State regulations, codes, and standards
- Ability to interpret regulations and make sound decisions
- Excellent communication and organizational skills
- Familiarity with FDA, EPA, and other regulatory organizations
- Ability to develop plans for regulatory compliance
- Proficiency with document management systems
- Proficiency with software applications such as Microsoft Word, Excel, and Access
- Ability to conduct research and analyze data
- Understanding of the product development process
- Experience with audits and quality assurance processes
- Ability to work independently or as part of a team
- Strong problem solving and decision-making skills
What skills should I put on my resume for Regulatory Affairs Specialist?
When writing a resume for a Regulatory Affairs Specialist role, it is important to emphasize the skills necessary for the position. Regulation-related positions require a wide range of skills and knowledge, including understanding of local and international laws, policies, and procedures.
If you are looking to apply for a Regulatory Affairs Specialist role, here are some of the key skills to include on your resume:
- Strong Regulatory Knowledge: As a Regulatory Affairs Specialist, it is important to have a deep understanding of local and international laws, policies, and procedures related to regulatory affairs.
- Attention to Detail: A good Regulatory Affairs Specialist needs to be detail-oriented and have excellent observational and analytical skills.
- Communication Skills: A Regulatory Affairs Specialist must be able to effectively communicate with a variety of stakeholders, such as regulatory authorities and other parties involved in the regulatory process.
- Technical Writing: A Regulatory Affairs Specialist must be able to create comprehensive and accurate documents to support regulatory submissions.
- Project Management: A Regulatory Affairs Specialist must be able to manage multiple projects and activities at once and work within deadlines.
- Problem-Solving: A Regulatory Affairs Specialist must be able to identify, analyze, and solve problems in a timely and efficient manner.
Incorporating these skills into your resume will show employers that you have the necessary qualifications to successfully fulfill a Regulatory Affairs Specialist role.
Key takeaways for an Regulatory Affairs Specialist resume
If you are looking to get started in the field of Regulatory Affairs, the best way to do that is to have an eye-catching, up-to-date resume. As a Regulatory Affairs Specialist, you will be responsible for ensuring that company products are compliant with regulatory requirements and industry standards. Therefore, it is important to make sure your resume is tailored to highlight key aspects of your experience that are relevant to the role. Here are some key takeaways for crafting a successful Regulatory Affairs Specialist resume.
First, make sure you list any relevant certifications or qualifications you have earned. This will demonstrate to potential employers that you have the knowledge and experience necessary for the job. Additionally, you should include any experience you have working with government regulatory agencies. This will demonstrate that you are familiar with the regulatory process and can effectively navigate it.
Second, highlight any experience you have working with product development, testing, and documentation. This will demonstrate that you have the expertise to ensure that products meet all applicable standards. Also, be sure to include any experience you have overseeing the transfer of products from clinical trials to commercial launches. This will show potential employers that you have experience getting products to market.
Finally, make sure you list out any experience you have working with product labeling, adverse event reporting, and recall procedures. This will demonstrate that you are knowledgeable of the regulatory process and can ensure that products remain compliant. Additionally, you should include any experience you have presenting to internal or external stakeholders. This will demonstrate that you have the communication skills necessary to effectively represent the company and its products.
By following these key takeaways, you can create an effective Regulatory Affairs Specialist resume that will help you stand out to potential employers. Make sure you take the time to tailor your resume to the role and highlight any relevant experience that you have. With the right resume, you can effectively demonstrate that you have the qualifications and expertise necessary to succeed in this role.
Let us help you build
your Resume!
Make your resume more organized and attractive with our Resume Builder
